Background: No standard of care first line therapy has been established for treatment naive patients with mantle cell lymphoma (MCL). Limited options exist in the treatment armamentarium for patients who cannot tolerate induction therapy. Venetoclax (V) based regimens have been studied recently in retrospective studies and in early phase clinical trials. We aim to systematically review the evidence of efficacy and safety of V based regimens in mantle cell lymphoma.
Methods: We carried out a systematic search on MEDLINE, Cochrane, ASH and ASCO meetings library. Screening was done to include studies with MCL patients who were treated with V monotherapy or any combination. Retrospective studies, case series, reports and reviews were excluded. Quality assessment was performed using methodological index for non-randomized studies (MINORS) tool. The primary efficacy outcomes evaluated included complete response (CR) by Lugano criteria, minimal residual disease (MRD) by flow cytometry, PCR or next generation sequencing (NGS). Safety outcomes evaluated included grade 3 or more adverse events including neutropenia and any tumor lysis syndrome (TLS) as assessed by common terminology criteria for adverse events (CTCAE) version 4. Predefined subgroup analysis was performed based on studies with first line vs RR disease and concurrent bruton tyrosine kinase inhibitor (BTKi) use vs not. Random effects model was used. Data was analyzed using R version 4.2.1.
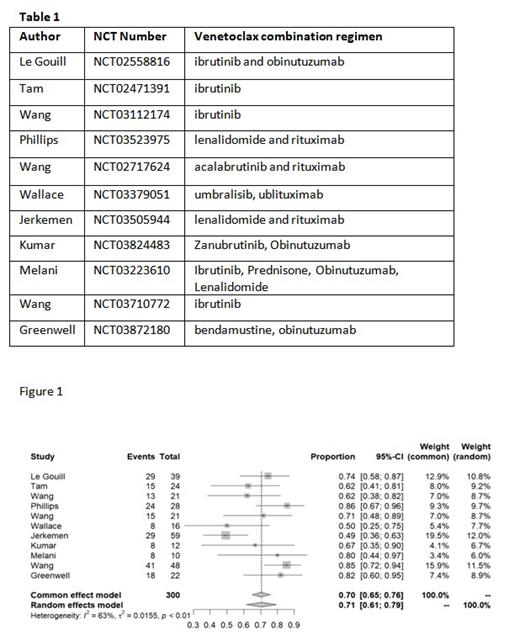
Results: 11 clinical trials with 300 patients were included. 5 out of 11 trials had V based regimen evaluated in treatment naïve population only while 3 trials evaluated only relapse refractory population. MRD assessment data was reported in 7 studies. V based combination regimens used in the trials are listed in table 1 below. Meta-analysis of 300 evaluable patients from 11 trials showed a pooled CR rate of 71% (95% CI: 61- 79%) with high heterogeneity (I 2 = 62%, p value 0.001)(figure 1). Pooled CR rate among studies with only treatment naïve patients was 82% (95% CI: 74 - 88%) with low heterogeneity (I 2 = 0%, p = 0.45) compared to other studies with pooled CR rate 62% (95% CI: 51 - 72%, I 2 = 41%, p 0.13) with significant difference between the two subsets by testing of moderator coefficient (p 0.009). However, there was no difference in CR outcomes noted based on studies conducted with concurrent (BTKi) use (p 0.7). Pooled analysis from 7 studies reporting MRD data revealed an overall MRD rate of 67%(95% CI: 56 - 78%) with high heterogeneity(I 2 = 57%, p 0.02). Pooled grade 3 or more neutropenia rate was 35%(95% CI: 14 - 58%, I 2 = 88%, p< 0.01). The overall TLS rate among 9 reporting studies was 3.1%(95% CI: 0.2- 7.9%, I 2 = 46.7%, p 0.06).
Conclusions: V is a viable option as part of treatment regimen for MCL patients with high response rates among treatment naïve patients as well and modest response rates among patients with relapse refractory disease. There is considerable heterogeneity of MRD rates accross studies, due in part to differences in study populations and to methodology of MRD testing. Pooled neutropenia and TLS rates appear to be low and make V a safe option as part of regimen.
Disclosures
Diaz Duque:Morphosys: Consultancy; Genentech: Consultancy; Lilly: Consultancy; ADCT: Consultancy; AstraZeneca, ADCT, Lilly, Morphosys, Genentech: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal